determination of sulphate in water by gravimetric method pdf|sulfate in an aqueous solution : broker GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown . WEBSuas aulas têm como foco ir além apenas do idioma. Você vai conhecer toda uma nova cultura diretamente com professores nativos e experientes no ensino, mergulhando mais no idioma. Aprenda espanhol online no .
{plog:ftitle_list}
25 de jun. de 2017 · 今天聊聊美剧《传教士第2季》。 片名Preacher Season 2 (2017)。 这是一部AMC从DC手里接过的漫改剧。 剧情紧接着第一季,暴力传教士杰西·卡斯特Jesse Custer在得知上帝失踪后,踏上了寻找上帝的旅程。
The analysis method was based on a suppressed conductivity system that simultaneously analyzed the anionic macroelements on Metrohm Supp A 250/4.0 column .In this study, two simple methods (conductometric titration and .GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown .
towel thickness measurement
In this study, two simple methods (conductometric titration and turbidimetric method) have been used for the quantitative determination of sulphate ions from aqueous media.This Internationa! Standard specifies a gravimetric method for the determination of sulfate in water. The method is applicable to the analysis of all types of water, including sea water and .
Sulphate Gravimetric Method for Sulphate Determination: Theory: Nearly all sulphates in water can be precipitated as BaSO 4 on reaction with barium chloride (BaCl 2) under acidic .The gravimetric method of sulphate ions determination is based on the precipitation reaction with BaCl2: SO4 2- + BaCl2 BaSO4 + 2 Cl- (1) when a white precipitate of BaSO4 is formed, which .Gravimetric Determination of Soluble Sulfate. The quantitative determination of sulfate ions in inorganic compounds can be accomplished by using the selective precipitation of the sulfate .
GRAVIMETRIC DETERMINATION OF SULFATE IN A SOLUBLE SAMPLE The analysis of soluble sulfate is based upon its precipitation with barium ion. 2+ 2 Ba (aq) + SO 4 - (aq) → .Gravimetric methods: The quantitative methods that are based on determining the mass of a pure compound to which the. analyte is chemically related. Precipitation gravimetry: The .
Determination of Sulfate in drinking, surface and waste water. Description. the Sulfate is possible using the Ca-ISE and EGTA as titrant. An excess of . aCl2 is added to the water .Chemistry 321: Quantitative Analysis Lab note Gravimetric Analysis: Determination of % Sulfur in Fertilizer This is another "real world" sample experiment – in this case we will analyze a fertilizer sample for the sulfate content and express the result as %S. Fertilizers are often specified by their N, P, K content –Construction and application of a portable microcontrolled turbidimeter for the in situ determination of sulfate.pdf. Vagner Bezerra . method is described for the determination of sulfate in mine water. When the SO4 2− reacts with barium .carefully performed, the turbidimetric method provides reproducible results for such an important determination. This method is much faster and sensitive as compared to the commonly employed gravimetric determination. In the next experiment you would learn about the application of IR spectrometry in the determination of functional group
Unlike these, gravimetry is a chemical method of analysis, which is much easier to use, because require only common laboratory devices and does not involve high costs (Bejan et al., 2006). The gravimetric method of sulphate ions determination is based on the precipitation reaction with BaCl 2: SO 4 2-+ BaCl 2 -BaSO 4 + 2 Cl (1)of water content in uranium oxide by gravimetric method using a microprocessor oven. Water is physically absorbed by a solid material and does not form chemical bonds in a material can be realeased
The sulphate present in the different soil samples was extracted in the ratio of 1:1 using water. The sulphate anion was treated with excess barium chloride solution to produce the barium sulphate precipitate and spectrophotometric estimation of the same was carried out at 420 nm wavelength. . 1991). Gravimetric Determination of Sulphate .
Determination of Sulfate in drinking, surface and waste water . Description . The determination of theSulfate is possible using the CaISE and EGTA as titrant. An excess of BaCl2 is - added to the water sample to precipitate the sulfate. The not reacted Baions are back titrated with EGTA - titrant. The titration curve shows two equivalence points.for the determination of sulfate. Which indication method is the most suitable depends above all on the sample matrix. Method 1: Precipitation as barium sulfate and back-titration of the Ba2+ excess with EGTA. The ion-selective calcium electrode is used as indicator electrode. Method 2: As in method 1, but with the electrode combination .copper sulfate [CuSO4 xH2O] Features of Gravimetric Analysis •A given analyte is isolated from the sample and weighed in some pure form. •One of the most accurate and precise methods of macro quantitative analysis. •One of the oldest methods known (before 1810). •Absolute analysis (no standard needed).
Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . The change in the absorbent’s mass provides a direct determination of the amount of water in the sample. An easier approach is to weigh the sample of food before and after we heat it and use the change in its mass to determine . 3. Gravimetric method with drying of residue If organic matter is not present in the sample first method can be done without igniting and instead drying the residue and weighing. Turbidimetric method Turbidimetric method is method of measuring sulphate is based upon the fact that barium sulphate tends to precipitate in a colloidal form and that this tendency is .Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed .
Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . to determine the sulphate ions (SO 4 2-) contained in ammonium . water, and sulfur dioxide. This method has been successfully used to estimate a wider range of organic compounds, including lactose in milk .4500-SO42− A. Introduction 1. Occurrence Sulfate (SO42−) is widely distributed in nature and may be present in natural waters in concentrations ranging from a few to several thousand milligrams per liter. Mine drainage wastes may contribute large amounts of SO42− through pyrite oxidation. Sodium and magnesium sulfate exert a cathartic action.What Is Gravimetric Analysis GRAVIMETRIC ANALYSIS In precipitation gravimetry, the analyte is converted to a sparingly soluble precipitate. This precipitate is then filtered, washed free of impurities, converted to a product of known composition by suitable heat treatment, and weighed. For example, a precipitation method for
Sulfate in aqueous solutions may be determined by a gravimetric method in which sulfate is precipitated as barium sulfate; the method is suitable for sulfate . 3.2 Water Sulfate concentrations in rain in Canada ranged between 1.0 and 3.8 mg/litre in 1980 (Franklin et al., 1985). An annual mean value of about 6 mg/litre in precipitation over
Introduction Gravimetric analysis is a technique through which the amount of an analyte (the ion being analyzed) can be determined through the measurement of mass. Gravimetric analyses depend on comparing the masses of two compounds containing the analyte. The principle behind gravimetric analysis is that the mass of an ion in a pure compound can be determined and . The classic technique for sulfate analysis in an undergraduate quantitative analysis lab involves precipitation as the barium salt with barium chloride, collection of the precipitate by gravity .
12 Gravimetric Methods of Analysis Gravimetric analysis: based upon mass measurements highly accurate and precise. --- Isolation (pure known substance) & weighing *Advantages 1. accuracy 2. single fundamental chemical precipitation reaction ex: Cl- + Ag+ → AgCl(s) 3. require no accuracy known standard solution 12A Precipitation Gravimetry 1.The study examines the groundwater quality of Kaduna south Local government area. The physio-chemical analysis of PH, sulphate, Phosphate, chloride, Iron, Electrical conductivity, Turbidity, Nitrates, Total suspended solid and Total hardness were carried out, the result obtained were compared with World Health Organization (WHO) recommended standards for drinking .METHOD 375.2. DETERMINATION OF SULFATE BY AUTOMATED COLORIMETRY. Edited by James W. O'Dell Inorganic Chemistry Branch Chemistry Research Division. Revision 2.0 . Dilution Water: Add 0.75 mL of sulfate stock solution (Section 7.10) and three drops of Brij-35 (CASRN 9002-92-0) to 2 L of reagent water.Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte . The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H 2 C 2 O 4, . The calcium sulfate (CaSO 4) in the tube retains carbon .
The classical gravimetric methods for sulphate determination are certainly more accurate and precise, but simplicity, cheapness and rapidity are advantageous characteristics of this titrimetric type of determination of sulphate (and other very .Method Summary A water sample is acidified to pH < 2 and extracted three times with hexane (or “hexanes”) in a separatory funnel. The extract is dried with sodium sulfate, the solvent and any volatile components are evaporated, and the residue is weighed with a 4-place balance. . prior to gravimetric determination of the residue using at .Gravimetric Analysis as one of the Analytical method for the Quantitative Determination of sulphate ions from aqueous media being the most simplest, rapid and low-cost method. This study evaluates some selected parameters and the results were
Gravimetric Analysis as one of the Analytical method for the Quantitative Determination of sulphate ions from aqueous media being the most simplest, rapid and low-cost method. This study evaluates some selected parameters and the results were compared with some regulatory standard; Federal Ministry of Environment (FME) in Nigeria and the World .Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. . and aPrecipitation method The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H 2 .
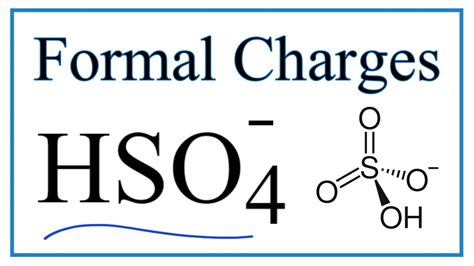
sulfate to hydrogen sulfide formula
Resultado da 15 de jul. de 2023 · 总算出来了! 去年11月初拍摄的超级大作,今天总算和大家见面,三上悠亚配上新ありな(新有菜)及相沢みなみ(相泽南),三位FANZAアダルトアワード(FANZA成人大赏)最优秀女艺人的组合,就是5月最豪华的共演作品〜 只是要和大家说声抱歉,当时我只知道是三人共演,但并不清楚主 .
determination of sulphate in water by gravimetric method pdf|sulfate in an aqueous solution